STI visits are an opportunity for an HIV prevention discussion—start the conversation today
STI history is one of the strongest indicators of potentially acquiring HIV1-3

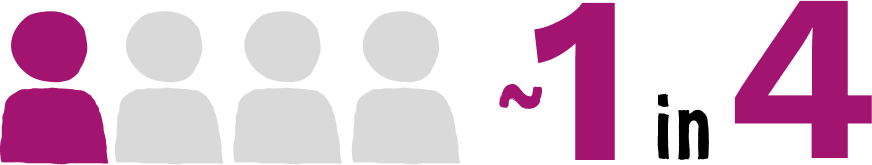
individuals recently diagnosed with HIV had an STI within the 6 months prior to their diagnosis2
(Truong HM, 2015; N=214. Study analyzed cases between 2005 and 2011 at publicly funded and community-based clinics in San Francisco)
Bacterial STIs have continued to increase significantly over the last 20 years4
From 2000 to 2023:
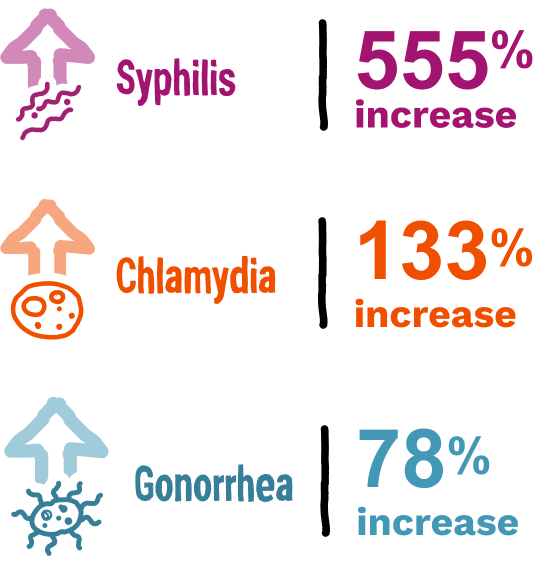

In 2023 in the US, there were more than 2.4 million STI cases.5
Certain groups continue to be disproportionately affected by STIs (including HIV)6,7:
- People aged 15 to 24 years
- Gay and bisexual MSM
- Transgender women
- Some racial and ethnic minority groups
The CDC recommends that:
ALL
sexually active adults and adolescents
should be informed about PrEP for HIV prevention8
Integrate HIV prevention discussions at every STI visit8
Consider PrEP for people of any sexual orientation and relationship status, as appropriate, and ask if they have or have had:

A bacterial STI (eg, syphilis, gonorrhea, or chlamydia) in the past 6 months

An HIV-positive sexual partner with unknown or detectable viral load

One or more sexual partners of unknown HIV status and inconsistent condom use
Offer PrEP to people who ask for it, even if no risk factors are identified through appropriate assessments.
PrEP medication should be considered part of a comprehensive STI prevention plan; PrEP medications do not reduce the risk of STIs other than HIV.8,9
Many clinical practice guidelines have a consistent recommendation for proactive discussions with sexually active adults and adolescents about PrEP8,10,11
When talking about sexual history, asking all people the same essential questions helps remove stigma and opens the door for a PrEP conversation12:
Sexual activity and relationships
- Do you have any questions or concerns about your sexual health or HIV?
- Have you had sexual intercourse in the past 6 months?
- Is your sexual partner(s) new or long-term?
- Is your sexual relationship casual or monogamous?
- Are you and your partner(s) in an open relationship?
- Do you have 1 or more sexual partners of unknown HIV status?
STI history
- What do you do to protect yourself from STIs, including HIV?
- Have you or your partner(s) ever had an STI?
- If you use STI prevention tools (eg, condoms), what methods do you use, and how often?
- Have you ever been tested for STIs? Would you like to be tested?
- How consistently do you use condoms?
Previous PrEP use
- Are you familiar with any of the methods to help prevent HIV, such as pre-exposure prophylaxis?
- Have you ever been on PrEP, a medication for HIV prevention?
- Why did you stop taking PrEP?
- Would you be interested in learning about a way to help prevent HIV?
HIV testing habits
- Have you ever been tested for HIV? When was the last time you were tested? Would you like to be tested?
- Do you tend to get tested for HIV regularly, or just when you think you may have been exposed?
While these questions can help identify individuals who could benefit from PrEP, the CDC recommends that PrEP be prescribed to those who ask for it, even if no risk factors are identified after an appropriate assessment.8
REMEMBER
Simply asking to be screened for HIV or requesting an STI test signifies an opportunity to be counseled about PrEP.
Be the catalyst for a conversation. Discuss HIV prevention at every STI visit.
CDC=Centers for Disease Control and Prevention; MSM=men who have sex with men; STI=sexually transmitted infection.